I had planned on writing about something else this week, but late last week another story caught my eye. Because it serves as a perfect followup to what I wrote about last week. To recap, I wrote about a man named Jim Gass, a former chief legal counsel for Sylvania, who had suffered a debilitating stroke in 2009 that left him with a useless left arm and weak left leg. He could still walk with a cane, but was understandably desperate to try anything to be able to function more normally in life. Mr. Gass was both driven enough, credulous enough, and wealthy enough to spend $300,000 pursuing stem cell tourism in China, Mexico, and Argentina over the course of four years. The result is that he now has a tumor growing in his spinal column that, as reported in the New England Journal of Medicine (NEJM) and the New York Times (NYT). Genetic analysis has demonstrated that the cells in this tumor mass did not come from Jim Gass, and the mass has left him paralyzed from the neck down, except for his right arm, incontinent, and with severe chronic back pain. Worse, although radiation temporarily stopped the tumor from growing, apparently it’s growing again, and no one seems to know how to stop it. Given that what makes stem cells so desirable as a regenerative treatment is their plasticity and immortality (ability to divide indefinitely) are shared with cancer, scientists doing legitimate stem cell research have always tried to take precautions to stop just this sort of thing from happening in clinical trials. Clearly, “stem cell tourist” clinics, which intentionally operate in countries where the regulatory environment is—shall we say?—less than rigorous are nowhere near as cautious, as they charge tens of thousands of dollars a pop for stem cell treatments that might or might not actually have real stem cells in them.
At the time I wrote that article, I emphasized primarily clinics outside of the US, where shady operators locate in order to be able to operate largely unhindered by local governments. You’d think that such a thing couldn’t possibly be going on in the US. You’d be wrong. Last week, Paul Knoepfler, a stem cell scientist who has previously contributed to Science-Based Medicine, teamed up with Leigh Turner to publish a paper in Cell Stem Cell estimating the number of stem cell clinics in the US. The number they came up with astonished me.
Stem cell clinics: A large and growing industry
In their article, Selling Stem Cells in the USA: Assessing the Direct-to-Consumer Industry Turner and Knoepfler explain:
Businesses marketing putative stem cell interventions have proliferated across the U.S. This commercial activity generates a host of serious ethical, scientific, legal, regulatory, and policy concerns. Perhaps the most obvious regulatory question is whether businesses advertising nonhomologous autologous, allogeneic, “induced pluripotent,” or xenogeneic “stem cell therapies” are exposing their clients to noncompliant cell-based interventions. Such practices also prompt ethical concerns about the safety and efficacy of marketed interventions, accuracy in advertising, the quality of informed consent, and the exposure of vulnerable individuals to unjustifiable risks.
Prior analyses of companies engaged in direct-to-consumer marketing of stem cell interventions have not explicitly focused on attempting to comprehensively locate and examine U.S. businesses (Lau et al., 2008, Ogbogu et al., 2013, Regenberg et al., 2009), although recent scholarship has identified some U.S. businesses engaged in such activity (Connolly et al., 2014). While such companies have attracted some scrutiny from researchers and journalists, these businesses have not yet been examined in a comprehensive manner (Perrone, 2015, Turner, 2015a). This gap in scholarship has contributed to misunderstandings that need to be corrected.
For example, health researchers, policy-makers, patient advocacy groups, and reporters often use the phrase “stem cell tourism” when addressing the subject of unapproved cell-based interventions and even in 2016 assume that U.S. citizens must travel to such destinations as China, India, Mexico, and the Caribbean if they wish to access businesses promoting stem cell procedures for a wide range of clinical indications. While travel from the U.S. to international “stem cell clinics” continues, the rhetoric of “stem cell tourism” often fails to acknowledge the hundreds of U.S. businesses engaged in direct-to-consumer advertising of stem cell interventions.
Of course, I did exactly this in my previous post, not really acknowledging this industry in the US. True, I did mention the San Diego-based company Stemedica, but I mentioned that company mainly because its business model appears to involve doing actual FDA-monitored clinical trials of its stem cell products in the US but referring any patients contacting the company who are ineligible for its US clinical trials to one of its foreign partners, particularly its affiliate right across border in Tijuana, Novastem. It was, for example, Novastem through which Gordie Howe was treated for his stroke a year and a half ago. Patients referred to Stemedica’s partners, of course, pay full price for the stem cell injections, usually around $30,000 a pop.
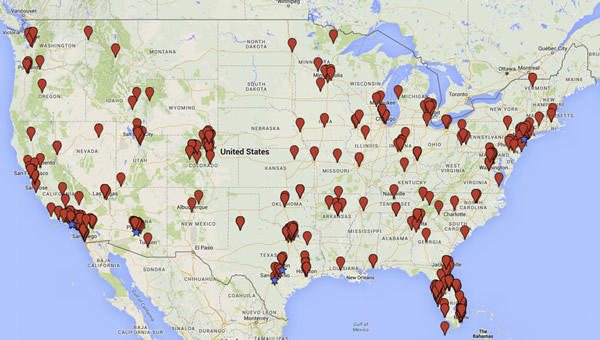
But what about US-based businesses? Turner and Knoepfler used key words and phrases such as “stem cell treatment” and “stem cell therapy” to preliminarily identify putative stem cell businesses and then evaluated the text on the websites of these businesses to refine their analysis. As a result, they identified 351 businesses offering stem cell therapies at 570 clinics, which they listed on this map:
They also helpfully include a link to an Excel spreadsheet listing all 570 sites, noting:
Many stem cell companies employ multiple physicians and advertise interventions available at numerous clinics. Although such businesses are widely distributed all over the county, we found that clinics tend to cluster in particular states. For example, we found 113 clinics in California, 104 in Florida, 71 in Texas, 37 in Colorado, 36 in Arizona, and 21 in New York. “Hotspot” cities including Beverly Hills (18), New York (14), San Antonio (13), Los Angeles (12), Austin (11), Scottsdale (11), and Phoenix (10) are designated with stars on the map. Some metropolitan areas, including Southern California around Los Angeles and San Diego, the South Florida region surrounding Miami, the greater Denver area, and the Dallas-Fort Worth metro region, have a relatively high number of clinics even if not all such facilities are technically in one city (Figure S1). While our analyses here do not explain why these businesses cluster in particular areas, we plan to investigate this question further. Possible factors include a relationship between number of clinics and population density, regional variations in use of “alternative” medical interventions, aging population demographics, and regulatory orientation of state medical boards and consumer protection agencies.
I’m sure population density has something to do with so many clinics in California, although one would expect more in New York and Texas if it were just population density. I also suspect that the prevalence and popularity of alternative medicine practitioners has something to do with it, given that, oddly enough, I frequently see ads for stem cell clinics and articles praising stem cell therapies on websites oriented towards alternative medicine. Given how often stem cells are advertised as “anti-aging” treatments (something mentioned by Turner and Knoepfler) and the popularity of plastic surgery in California, it wouldn’t surprise me if there is a correlation there as well. These are definitely things that I hope Turner and Knoepfler will look at in future investigations.
So what are these clinics selling stem cells to treat? What are the claims they make? Unfortunately, the claims of US clinics are not much, if at all, different from the claims made by many stem cell tourist clinics in other countries. Claims are made that specific diseases can be treated that are just specific enough to attract customers but vague enough not to promise too much.
Claims, claims, claims, and more claims
It has to be noted that there is not just one kind of stem cell. As I described last time, there are multiple kinds of stem cells, ranging from embryonic stem cells that require human embryos to isolate to adult stem cells to cells induced to be stem cells by the introduction of genes responsible for maintaining the “stem cell” state. As I’ll discuss later, this matters when it comes to asking just what the heck the FDA is or isn’t doing about this proliferation of stem cell businesses.
Turner and Knoepfler note that most of the businesses that they identified market autologous stem cell-based interventions; i.e., stem cells isolated from the patient and then reinfused. Most isolated these stem cells (or claimed to isolate them, given that it’s not always clear how such clinics verify that what they have isolated are indeed autologous stem cells) from adipose tissue (fat) or from the bone marrow. Be that as it may, 61% of the clinics examined market autologous adipose-derived stem cell-based interventions; 48% what they describe as autologous stem cells obtained from bone marrow; and 4% stem cells reportedly isolated from peripheral blood. Not surprisingly, lots of clinics offer more stem cells isolated from more than one source. Some offer “mixed” stem cells from both bone marrow and adipose tissue as “combination stem cell therapy.”
About one in five clinics advertised allogeneic stem cell treatments; i.e., stem cells from another person or source. The usual sources of these “stem cells” are advertised as amniotic material/fluid (17%), placenta (3.4%), and umbilical cords (0.6%). It’s noted in the report the precise source for these products was not clear in all cases, in particular for amniotic stem cells. Indeed, one wonders (at least I do) what the source of amniotic fluid is from which these clinics claim to isolate stem cells. Do they have a deal with a local obstetrical clinic or hospital to provide amniotic fluid or membranes? Do they buy placentas and amniotic membranes from a hospital? Where do these clinics get the raw material (i.e., the human tissue and fluids) to generate these stem cells from? Inquiring minds want to know!
Turner and Knoepfler also noted one business that offers what it claims to be induced pluripotent stem cells. (Remember, these are cells genetically manipulated to revert to being stem cells.) I went back to the spreadsheet and found which company offered this, Regenerative Medical Group. RMG claims to provide “induced pluri-potent stem cells from your own cells via an affiliated laboratory,” but what I found more interesting were the diseases and conditions it claims to treat with stem cells. Not surprisingly, as was the case for most of the clinics listed, many of the indications were orthopedic, to regenerate cartilage and repair injury. However, RMG also claims to be able to treat kidney diseases, macular degeneration, Parkinson’s disease, and, yes, autism. Under a tagline of An autism therapy that WORKS, there’s even a video on the website that makes claims that can only be described as grandiose and not supported by science featuring Bryn J. Henderson, DO, JD, FACPE, CIME, the executive director of RMG:
[embedded content]
In the video, Dr. Henderson claims that RMG has helped “dozens” of children with autism using stem cells. He claims that the stem cells circulate through the body, cross the blood-brain barrier and “make new cells” that change the course and prognosis of the patient with autism. He even claims that most of the time, the change is major. How does he know? He brags about the thank you cards he’s gotten from parents. I mean, seriously. This is utterly pathetic Even antivaccine quacks like Mark and David Geier or Andrew Wakefield can do better providing evidence. Note that that is not a compliment, given how poor their attempts at studies invariably are. Dr. Henderson, however, presents no science, no clinical trials, no preclinical trials, no nothing other than testimonials, although he does use a lot of science-y-sounding terms. Hell, I’ve seen homeopaths who provide more evidence and a more convincing presentation. At least they will cite actual patients rather than thank you notes from patients’ families. (Oh, and Dr. Henderson, don’t bother taking your video down; I’ve downloaded it.)
RMG’s fact sheet on autism is no better. Citing no evidence and not even case reports, the sheet claims that stem cell infusions for autism can improve:
- Social interaction
- Sensory problems
- Seizures
- Mental health issues
- Mental impairment
- Digestive issues
- Sleep disturbances
- Aggression
What evidence is presented? Again, none. This might as well be a chelation therapy clinic. The treatment, however, takes three days, and the patient doesn’t have to come back to an RMG clinic at all, although, the fact sheet hastens to add, they can undergo repeat treatments if necessary. In other words, stem cells appear to have been added to the armamentarium of “autism biomed” quackery.
I could go on, but to me stem cells for autism is so obviously dubious at best and bogus at worst that, given my interest in vaccines and the antivaccine movement’s mistaken belief that vaccines cause autism, I hope you’ll forgive me if I zeroed right in on autism. Indeed, nine of the clinics listed in the spreadsheet claim to be able to use stem cells of one kind or another to treat autism.
But, wait, there’s more. In addition to RMG:
Another business markets access to what it describes as “embryonic stem cell” interventions. In addition, we identified two clinics that marketed “bovine amniotic cells,” a xenogeneic product, for use in humans. Approximately 3% of businesses marketed stem cell interventions without mentioning a particular type of stem cells.
Perusing the list of clinics, I found it hard not to come to the conclusion that there isn’t a single disease or condition that someone, somewhere, isn’t claiming can be helped with stem cells of one kind or another. Diabetes, heart disease, degenerative diseases, Parkinson’s disease, Alzheimer’s disease, spinal cord injuries, stroke, aging, and even cancer show up on the list of conditions that these 570 clinics claim to be able to treat with stem cells, as Turner and Knoepfler note:
U.S. businesses promoting stem cell interventions claim to treat a wide range of diseases and injuries, as well as advertising stem cells for cosmetic applications, “anti-aging,” and other purposes (Figure 2B). Some clinics occupy relatively specialized marketplace niches. For example, many cosmetic surgery clinics advertise such procedures as “stem cell facelifts” and “stem cell breast augmentation” as well as sexual enhancement procedures. Orthopedic and sports medicine clinics often promote stem cell interventions for joints and soft tissue injuries. Other clinics take a much broader approach and list stem cell interventions for 30 or more diseases and injuries. Such businesses commonly market treatments for neurological disorders and other degenerative conditions, spinal cord injuries, immunological conditions, cardiac diseases, pulmonary disorders, ophthalmological diseases and injuries, and urological diseases as well as cosmetic indications. Many of these marketing claims raise significant ethical issues given the lack of peer-reviewed evidence that advertised stem cell interventions are safe and efficacious for the treatment of particular diseases. Such promotional claims also generate regulatory concerns due to apparent noncompliance with federal regulations.
Unfortunately, these US businesses are less unlike the stem cell tourist clinics that I’ve written about
Where’s the FDA?
I thought about perusing the list of clinics in more detail and picking out the most egregious examples other than RMG, but that can wait for a potential future post. (It is, after all, a holiday weekend, and we’ll be having visitors.) So instead I’ll move on to conclude with the question that many of you are probably wondering after seeing an example such as treating autism with stem cells: What the heck is the FDA doing?
This isn’t as simple as it sounds. For one thing, as noted in Turner and Knoepfler’s supplemental methods section:
However, it should be noted that according to 21 CFR 1271.3 (d) (4), minimally manipulated bone marrow for homologous use does not require pre-marketing approval by the FDA. 21 CFR 1271.15 (b) states that facilities removing cells or tissues from an individual and implanting those cells or tissues in the same individual during the same surgical procedure likewise do not require premarketing approval. In addition, federal regulations contain detailed criteria specifying when autologous or allogeneic cells can be used without first obtaining FDA premarketing approval. These criteria are identified in 21 CFR 1271.10. We mention these important sections of 21 CFR 1271 for a reason. Our goal was to identify businesses that engage in direct-to-consumer marketing of stem cell interventions and fit within our inclusion criteria. Judgments about regulatory compliance or noncompliance had no bearing on whether specific businesses were included in our database. Federal regulations governing marketing, manufacture, administration, and registration of cell-based interventions are complex, products are classified into different risk- based regulatory tiers, and we in no way wish to claim or imply that inclusion of particular businesses in Supplemental Table 1 means that they are noncompliant with federal regulations. Such determinations, as well as other assessments of regulatory compliance, must be made by legally authorized regulatory agencies after rigorous evaluation processes.
This is, of course, the reason why so many of these businesses offer—or claim to offer—bone marrow or adipose stem cells. If they don’t manipulate the cells too much, they can skirt FDA regulations, although the FDA is moving to crack down on unproven stem cell treatments and have started to issue warning letters. It’s a complex issue, but it’s hard not to look at the number of clinics and the breadth of health claims documented by Turner and Knoepfler and not come to the conclusion that there is a serious problem here. It’s also clear that big money and political interests are hindering the FDA. For example:
Some proponents of deregulation argue that current federal regulations governing the advertising, processing, and administration of autologous stem cells are too onerous and have resulted in few approved stem cell therapies reaching the American marketplace (Chirba and Garfield, 2011, McAllister et al., 2012). The REGROW Act is an example of the current push from some political quarters and even from some individual stem cell researchers for lowering safety and efficacy standards for adult stem cell-based interventions. However, we found that hundreds of U.S. businesses are already promoting stem cell interventions for an extraordinary range of clinical indications. Advocates of deregulation will perhaps be pleased by our findings that many putative stem cell interventions are currently available for sale in the U.S. In contrast, proponents of a marketplace in which cell-based therapies have traditionally been tested for safety and efficacy and subject to pre-marketing review by the FDA will likely be concerned by how many U.S. businesses are currently marketing stem cell interventions. We are particularly concerned that we found many advertising claims related to ALS, Alzheimer’s disease, Parkinson’s disease, and many other conditions for which there is no established scientific consensus that proven safe and efficacious stem cell treatments now exist.
The REGROW Act sounds a lot like the 21st Century Cures Act, ideologically-driven “solutions” that mistakenly argue that the way to let loose a torrent of cures for every disease imaginable is to “unleash” the power of the market through deregulation. In the case of the 21st Century Cures Act, its proponents propose to give the NIH a bit more money in return for weakening the FDA. It’s basically a solution to a nonexistent problem. The REGROW Act is cut from the same cloth, as it would allow provisional approval of stem cell therapies without phase III trials and establishing a conditional approval paradigm. Together with “right to try” laws, the REGROW Act and the 21st Century Cures Act are of a piece with a libertarian, free market-driven agenda to hamper government regulatory agencies.
Perusing some of the websites, I couldn’t help but notice how dubious stem cell therapies seem to have found a comfortable home in alternative medicine clinics. Perhaps the most blatant example I found was the Purety Family Medical Clinic, which advertises itself as “holistic medicine specialists for women, men, and pediatrics as well as prolotherapy, IV, ozone, chelation, HRT and FMT.” Right alongside stem cell injections for “badly injured or degenerated tissue,” Purety also offers chelation therapy for “heavy metal detoxification,” high dose vitamin C drips, ozone therapy for cancer, naturopathy, fecal transplants for a variety of illnesses, and, yes, homeopathy, The One Quackery to Rule Them All.
Unfortunately, given how potentially promising stem cell therapies are, right now they are tainted by association with quackery like that described above. Basically, stem cells are being sold as being every bit as magical as alternative medicine like homeopathy. However, as PZ Myers points out:
Stem cells are not magic. They are plastic cells that are pluripotent — they can differentiate into a variety of different tissues. But they need instructions and signals in order to develop in a constructive way, and the hard part is reconstructing environmental cues to shape their actions. They’re like Lego building blocks — you can build model spaceships or submarines or houses with them, and they have a lot of creative potential, but it’s not enough to just throw the Lego blocks into a bag and shake them really hard.
That’s what these stem cell clinics are doing, injecting stem cells and hoping they do their thing without knowing how the body induces them to do their thing, all while charging patients large sums of money for the privilege of being in what is in essence a poorly designed, poorly regulated clinical trial.
I don’t know about you, but if I were a legitimate advocate of stem cell therapies, I’d be very disturbed at how easily stem cell therapies are currently “integrated” with pure quackery like chelation therapy and homeopathy. Being so easily associated with clinics like Purety is not a good way to make stem cell treatments respectable, but it is a good way to make a lot of money if you aren’t that concerned with medical evidence or ethics.